NEWS&EVENT
Quality is our core our concept
《Advanced Materials》:Super absorbent aerogel!
Release time:
2025-04-12 00:00
Source:
1、 Research background
With the continuous growth of population and the persistence of water pollution, freshwater shortage has become a global challenge, threatening the sustainable development of humanity. It is predicted that by 2050, global freshwater demand may increase by 55%. Therefore, any efforts to increase access to freshwater will significantly improve the quality of human life. Due to the large amount of water in the atmosphere, atmospheric water harvesting (AWH) is expected to alleviate the current water shortage problem. Compared with traditional desalination or water purification technologies, AWH can achieve dispersed freshwater acquisition without being affected by geographical or hydrological conditions. Early strategies such as fog capture and dew collection were either limited by climate conditions or energy intensity, and therefore were not widely applicable feasible solutions. Recently, using adsorbents to extract water from the air has become a promising AWH technology due to its good environmental adaptability. Especially, adsorption based AWH (SAWH) with solar driven water release is more attractive in impoverished areas due to its low energy consumption and ease of operation.
The efficiency of SAWH mainly depends on the water absorption performance of the adsorbent. Conventional adsorbents, such as activated alumina, silica gel, and zeolite, either have low water absorption or can only release water at high temperatures. This has promoted the development of many new adsorbents such as hygroscopic salts, gel and metal organic frameworks (MOFs). Due to its high water absorption within a wide RH range and low cost, hygroscopic salts have become one of the most commonly used adsorbent materials for SAWH. But they have the disadvantages of deliquescence and agglomeration, leading to severe degradation of water absorption capacity and adsorption kinetics. Therefore, developing suitable and reasonably priced adsorbents is crucial for the practical application of SAWH. In addition to developing efficient adsorbents, it is also necessary to improve equipment levels to achieve continuous production of fresh water. Although SAWH devices have made significant progress in materials, their daily water production is still very low and far from reaching their potential. It is crucial to design the entire SAWH system from materials to equipment in order to achieve continuous and large-scale freshwater production.
2、 Research results
Adsorption based atmospheric water harvesting (SAWH) provides a sustainable strategy for addressing global freshwater scarcity. However, obtaining absorbents with excellent performance over a wide range of relative humidity (RH) and devices with fully autonomous water production capabilities remains challenging.
Recently, it has been reported abroad that magnesium chloride (MgCl2) has been innovatively converted into super hygroscopic magnesium complexes (MC). By coordinating hygroscopic salts with organic ligands in an unsaturated manner, the problems of salt deliquescence and agglomeration can be effectively solved. Then, MC combines with photothermal gas gel (composed of sodium alginate and carbon nanotubes (SA/CNTs)) to form composite gas gel, which shows high water absorption in a wide range of RH, reaching 5.43 and 0.27 kg kg-1 at 95% and 20% RH, respectively. The layered porous structure enables SA/CNTs/MC to exhibit rapid absorption/desorption kinetics, enabling 12 cycles per day at 70% RH, equivalent to a water production of 10.0L kg-1 day-1. In order to achieve continuous and practical freshwater production, an autonomous atmospheric water generator (AWG) with two SA/CNTs/MC absorption layers was designed and constructed, which can alternately perform water absorption/desorption processes. The device is fully automated, powered by solar energy, and does not require any other energy consumption, saving manpower and material resources. This study provides a promising approach for achieving autonomous, high-performance, and scalable SAWH.
3、 Research content
1. Synthesis and Characterization of SA/CNTs/MC Composite Aerogels
The preparation of SA/CNTs/MC composite aerogels mainly involves three processes: freeze drying, crosslinking and impregnation, as shown in Figure 2a. SA/CNTs aerogels were prepared by controlled freeze casting and subsequent freeze drying. In order to avoid structural collapse, the aerogel is immersed in CaCl2 solution, so that Ca2+can induce cross-linking. The size and shape of SA/CNTs aerogels can be optimized to meet different needs through different freezing molds. The crosslinked SA/CNTs aerogels show a uniform porous structure, with an average pore size of several microns and a smooth internal surface. The crosslinked SA/CNTs aerogels were impregnated with MC solution to produce SA/CNTs/MC composite aerogels (Fig. 2b).
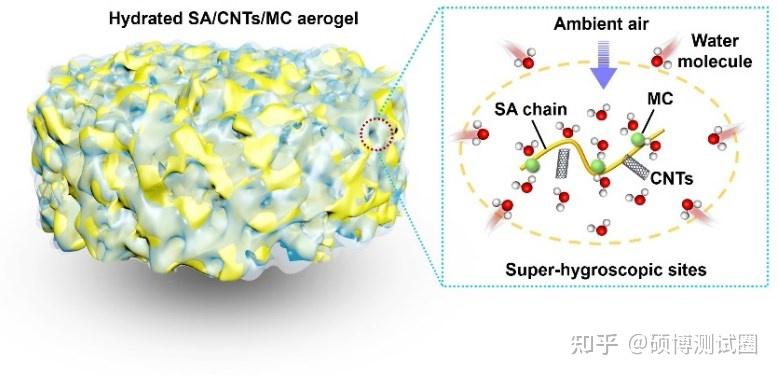
Figure 1. Design of super hygroscopic aerogel
Figure 2c shows that SA/CNTs/MC composite aerogels maintain a uniform porous structure, which means that the load of MC will not destroy the original structure. MC is evenly distributed on the SA sheet (Figure 2d). After being loaded onto SA/CNTs, there was no significant change in the morphology of MC (Figure 2e). The elemental diagram in Figure 2f indicates that the N, Mg, Cl, C, and O of MC are uniformly distributed on the SA sheet, further confirming the uniform distribution of MC particles. Figure 2g shows that the characteristic peaks of SA appear at 1417 and 1614cm-1, corresponding to the asymmetric and symmetric stretching modes of COO -, respectively. After modification with MC, the two peaks shifted to 1440cm-1 and 1631cm-1, respectively, indicating that there was an interaction between MC and SA, which was helpful to improve the stability of composite aerogels. As shown in Figure 2h, both SA/CNTs and SA/CNTs/MC exhibit H3 type isotherms. The BET surface area of SA/CNTs is 38.9m2 g-1, which can provide abundant sites for MC loading. After loading MC, the surface area decreased to 18.5m2 g-1. The pore volume decreased from 0.126cm3 g-1 to 0.054cm3 g-1, indicating that some pores were blocked. The pore size of SA/CNTs/MC is approximately 1.7-10nm, indicating a hierarchical porous structure. This unique structure can provide abundant water vapor channels, thereby achieving rapid water adsorption kinetics.
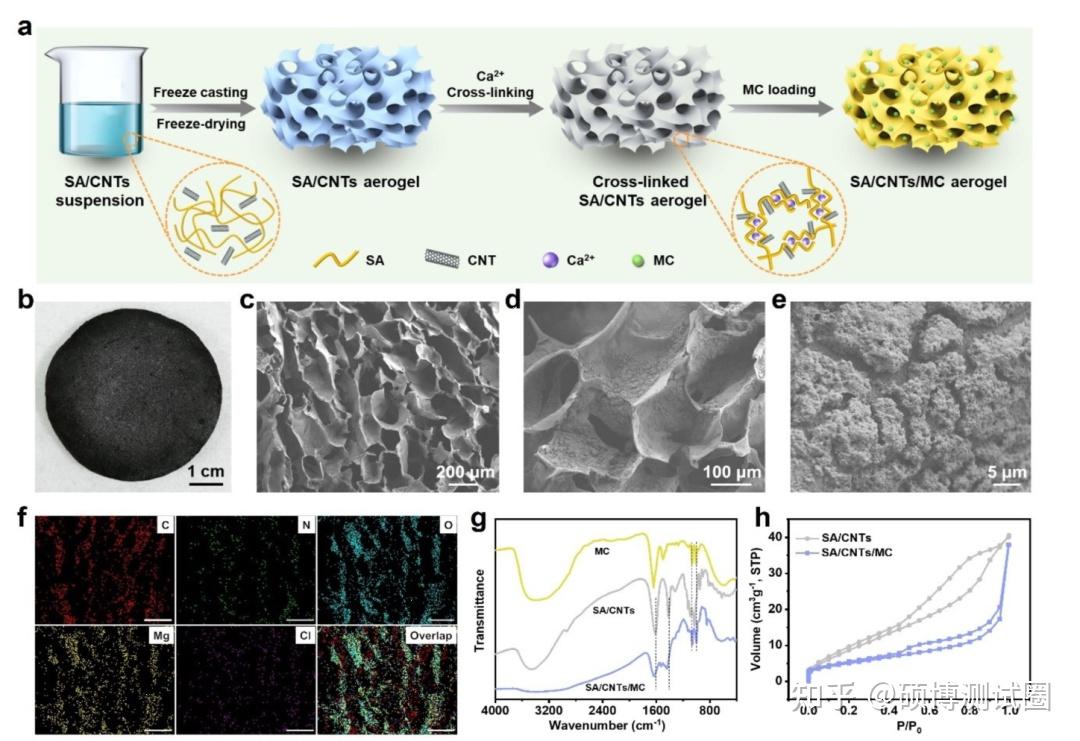
Figure 2. Synthesis and characterization of SA/CNTs/MC aerogels
2. Water absorption performance
As shown in Figures 3a and 3b, SA/CNTs/MC exhibited a rapid water absorption rate of 0.84 kg kg-1 h-1 within 1 hour under environmental conditions (24 º C, 70% RH), which was much higher than SA/CNTs (0.22 kg kg-1 h-1) and pure MC (0.56 kg kg-1 h-1). This can be attributed to the synergistic effect of SA and MC, that is, MC endows the aerogel with excellent moisture absorption, and the porous structure of SA provides rich transmission channels and expanded contact area for water vapor. In Figure 3c, SA/CNTs/MC exhibits almost consistent water absorption capacity at 20-40 ° C and shows a low regeneration temperature of approximately 60 ° C. It should be noted that SA/CNTs/MC exhibits excellent water absorption capacity over a wide range of RH, which can greatly broaden its applicability to different environments.
Figure 3d shows the effect of sample thickness on absorption kinetics, indicating that the absorption rate decreases with increasing sample thickness. In addition, dynamic water absorption was conducted at different temperatures, with a relative humidity maintained at 70% (Figure 3e), and the water absorption rate increased with increasing temperature. At 70% RH, SA/CNTs/MC require approximately 7, 5, and 3 hours to reach absorption equilibrium at temperatures of 20, 30, and 40 ° C, respectively. Therefore, higher temperatures provide faster dynamics. Under different RH conditions, the trend is the same (Figure 3f). Therefore, the higher the relative humidity, the faster the water absorption rate. In Figure 3g, water vapor is transported from the surrounding air to the dry SA/CNTs/MC aerogels, where it is captured and condensed into liquid water. Due to the hydrophilicity of the SA porous framework, the aerogel stores the absorbed water in an expanded form. As shown in Figure 3i, the volume of SA/CNTs/MC aerogels increases by about 38% after absorbing water vapor for 12h at 24 ° C and 90% RH, and the absorbed water can be released through solar radiation. Figure 3h records the real process of water drop growth and migration in SA/CNTs/MC. Smaller water drops gradually grow up, form larger water drops, and migrate to other parts of the aerogel.
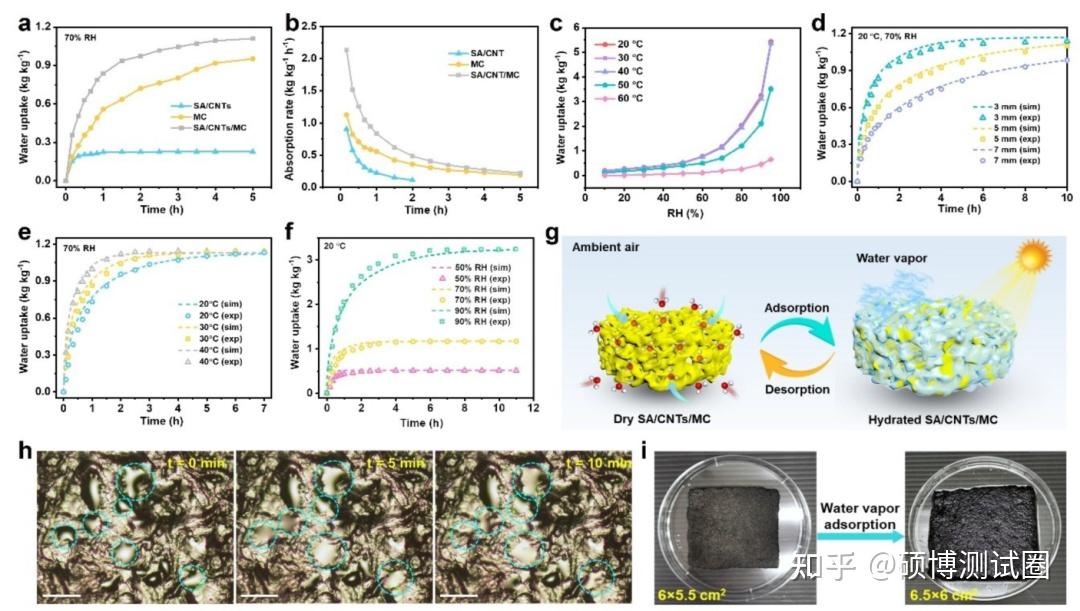
Figure 3. Water absorption performance of SA/CNTs/MC
3. Solar driven hydrolysis and absorption performance
SA/CNTs have a high solar absorption rate of~97% over a wide wavelength range (250-2500nm), which is much higher than pure SA (Figure 4a). Therefore, the surface temperature of SA/CNTs/MC increases rapidly within the first 20 minutes, and then slowly increases with the decrease of water content (Figure 4c). The equilibrium surface temperatures of SA/CNTs/MC at 70% RH and 90% RH can reach around 70.6 and 66.9 ° C, respectively (Figure 4b). SA/CNTs/MC saturated with water exhibited rapid hydrolysis and adsorption processes under one exposure to sunlight (Figure 4d). Figure 4e shows that when SA/CNTs/MC was treated with 70% RH and 90% RH, the maximum hydrolysis absorption rates were as high as 4.41 and 4.77 kg kg-1 h-1, respectively, which were significantly higher than other reported materials. As shown in Figure 4f, SA/CNTs/MC exhibits high cycling stability, and only ≤ 5% of the absorbed water cannot be desorbed after each cycle. Meanwhile, SA/CNTs/MC exhibited significant structural stability after multiple absorption/desorption cycles.
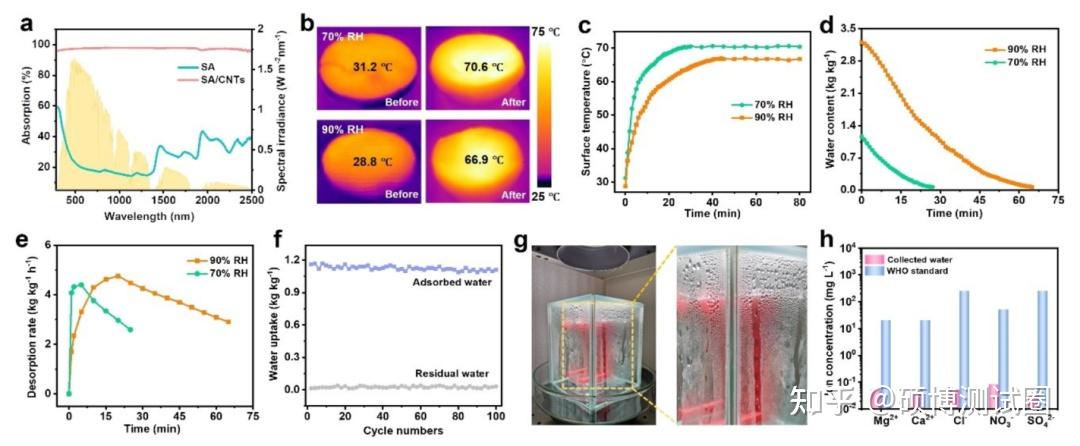
Figure 4. Hydrolytic adsorption performance of SA/CNTs/MC
4. Atmospheric water collection performance
Then assemble a simple device to demonstrate the solar powered freshwater production process. First, SA/CNTs/MC aerogels were treated with 90% RH for 12h, and then hydrolysis absorption experiments were carried out under one solar radiation. After the simulated light source is turned on, the absorbed water evaporates rapidly from the hydrated SA/CNTs/MC aerogels. Hot water vapor naturally condenses on the glass wall, and the production of fresh water is clearly manifested through the appearance of water droplets, condensation, and flow (Figure 4g). The water production of SA/CNTs/MC is 10.0 L kg-1 day-1, and the water collection efficiency is 89%. The concentration of all ions present in the sample is much lower than the World Health Organization standard, indicating that the collected water has the highest quality (Figure 4h). The collected water was further used for fish farming, and the movement of yellow croaker was monitored continuously for three days. The results showed that yellow croaker lived well in the collected water, confirming the good water quality.
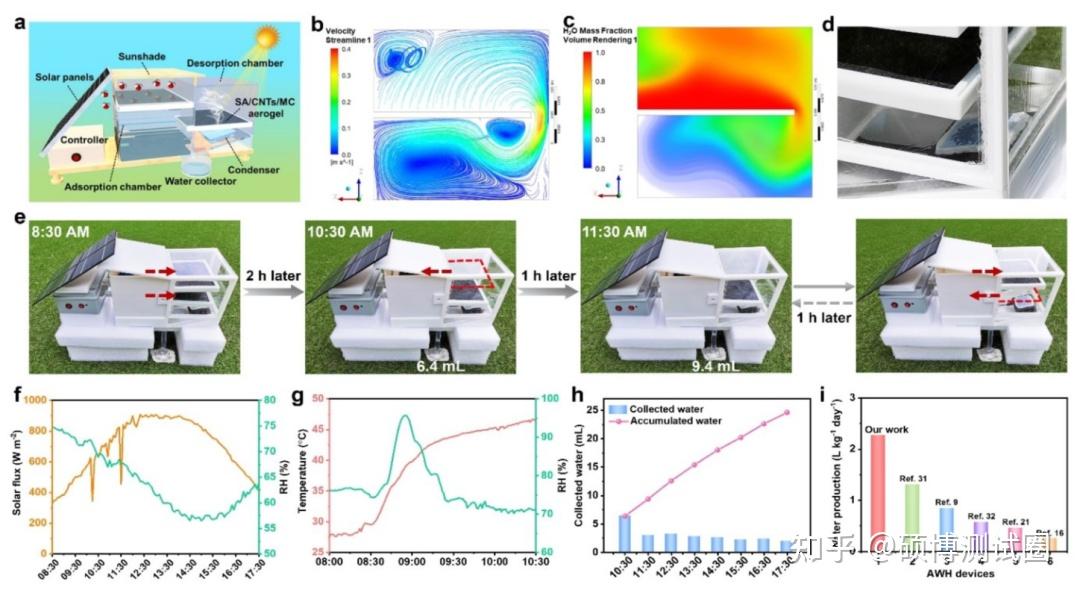
Figure 5. Schematic diagram of AWG
5. Solar powered Autonomous Atmospheric Water Generator (AWG)
In order to achieve continuous and large-scale freshwater production, researchers have designed and built a fully solar powered autonomous atmospheric water generator (AWG) with two absorption layers (one releasing water and the other capturing water). As shown in Figure 5a, the device mainly consists of four parts: absorption chamber, desorption chamber, control system, and solar energy system. The absorption chamber has a sun visor to avoid direct exposure of sunlight to SA/CNTs/MC aerogels during water capture. As shown in Figure 5b, water vapor tends to move from the upper part of the chamber to its lower area. Water vapor begins to precipitate in lower areas, which can be determined by an increase in water mass fraction (Figure 5c), which will promote water condensation at the bottom.
Water was captured overnight in the absorption chamber (average 80% RH), and at 8:30 AM the next day, both absorption layers autonomously moved to the desorption chamber for water release (Figure 5e). The solar flux and relative humidity of the environment were obtained from meteorological stations near the experimental site, as shown in Figure 5f. Once the absorption layer is exposed to sunlight (8:30 am), the temperature inside the desorption chamber rapidly increases (Figure 5g), causing the greenhouse effect and reducing the radiative heat loss of the desorption layer. In summary, the RH in the desorption chamber increased from approximately 74% to 96% within 20 minutes (Figure 5g), indicating a rapid hydrolysis and adsorption process. Figure 5d shows that at 11:30 am, a total of approximately 9.4 mL of water was collected. In this way, eight consecutive absorption desorption processes can be achieved in one day, collecting a total of approximately 24.6mL of water (Figure 5h), equivalent to a freshwater production of 1.64L kg-1 day-1. Under indoor and outdoor conditions, the water production of the equipment is much higher than previously reported equipment (Figure 5i). Note that this device has a wide range of applications and can be used together with other adsorbents, possessing more advantageous/tunable characteristics to further improve the performance of the device.
4、 Conclusion and Prospect
In summary, a comprehensive transformation of solar driven SAWH has been achieved from material preparation to device design. Firstly, innovatively converting MgCl2 into MC can effectively solve the problems of salt deliquescence and agglomeration. Then, SA/CNTs/MC composite aerogels with hierarchical porous structure were prepared, which showed excellent water vapor absorption capacity, rapid water absorption/desorption kinetics, easy regeneration and high cycle stability in a wide RH range. At relative humidity of 95% and 20%, the equilibrium water absorption rates of SA/CNTs/MC are as high as 5.43 and 0.27 kg kg-1, respectively. The most interesting thing is that sunlight can drive water release at a hydrolysis absorption rate of up to 4.77kg kg-1 h-1 (1 exposure to sunlight). To demonstrate the application, researchers designed and manufactured a fully solar driven autonomous AWG with two absorption layers that can alternate between water vapor absorption and desorption processes. This work has opened up a new path for the development of portable, low-cost, and high productivity solar adsorption AWH systems.
Previous


















