NEWS&EVENT
Quality is our core our concept
Nano Micro Letters Multifunctional Asymmetrical Double layer Aero gel! Efficient electromagnetic interference shielding and ultra-high frequency electromagnetic wave absorption
Release time:
2025-06-24 20:39
Source:
Introduction
Currently, electromagnetic radiation and pollution are becoming increasingly severe, and electronic devices are severely affected by electromagnetic interference (EMI), which is easily detected by multispectral detection technology. Traditional EMI shielding materials suffer from high reflection caused by impedance mismatch, and have a single function, making it difficult to meet the requirements of complex scenarios. MXene based aerogel has excellent conductivity and porous structure, but its single function limits its application. Therefore, it is urgent to design multifunctional materials that combine efficient electromagnetic wave absorption, broadband shielding, and multi scenario applicability. Through breakthroughs in structural design and preparation technology, the problems of reflection pollution and functional limitations of existing materials can be solved.
Based on this, this paper prepared lotion ink through electrostatic assembly of MXene/GO and octadecylamine (ODA), combined with 3D printing/molding to build an asymmetric double-layer aerogel: the top layer MG (MXene/GO) optimized impedance matching, and the bottom layer MXene enhanced reflection. This structure utilizes the absorption reflection reabsorption mechanism and interference cancellation effect to achieve an ultra-high absorption coefficient of 0.95 and a shielding effectiveness of>100 dB in the X-band, while maintaining an absorption rate of>0.9 in the 8.2-40 GHz ultra wideband. Aerogel has hydrophobic, thermal insulation, joule heating, solar thermal conversion and oil spill cleaning capabilities, providing innovative solutions for multi scene electromagnetic protection.
Experiment
The experimental part mainly includes material preparation, synthesis of GO and MXene, preparation of lotion ink, 3D printing and molding, and multi-dimensional performance characterization:
1. Materials: Using reagents such as octadecylamine (ODA), toluene, lithium fluoride (LiF, 99.99%), Ti ∝ AlC ₂ (400 mesh), sulfuric acid (98%), hydrochloric acid (37%), hydrogen peroxide (30%), etc. GO was synthesized from graphite flakes by improving the Hummers method, and MXene was obtained by etching Ti ∝ AlC ₂ with HCl/LiF.
2. Preparation of GO and MXene: GO suspension concentration is 7.5 mg/mL; When preparing MXene, Ti ∝ AlC ₂ was added to HCl/LiF solution for etching. After washing, centrifugation (3500 r/min), and ultrasonic exfoliation, MXene sheets with an average size of about 756 nm were obtained.
3. Preparation of lotion ink: MXene/GO suspension and ODA toluene solution (15 mg/mL) were emulsified at 1500-3000 r/min for 10 min to prepare MG lotion ink (MXGy, x, y are MXene/GO volume ratio) and MXene lotion ink with water phase volume fraction of 0.6-0.71.
4. 3D printing and molding: using a dual needle printer, the 410 μ m nozzle first prints the MG layer, then prints the MXene layer, and freeze-drying at -10 ℃ forms a double-layer aerogel (MGaL MXenebL); During molding, the ink is poured into the PTFE mold, and the MGc MXened aerogels with different thickness are also obtained by freeze-drying.
5. Performance characterization: Microscopic structure was observed by SEM/TEM, composition was analyzed by XRD/FT-IR, hydrophobicity was measured by contact angle tester, electromagnetic shielding performance was tested by vector network analyzer (8.2-40 GHz), thermal response was recorded by infrared camera, and thermal insulation was tested by thermal conductivity meter according to ISO 22007-2 standard.
Discuss
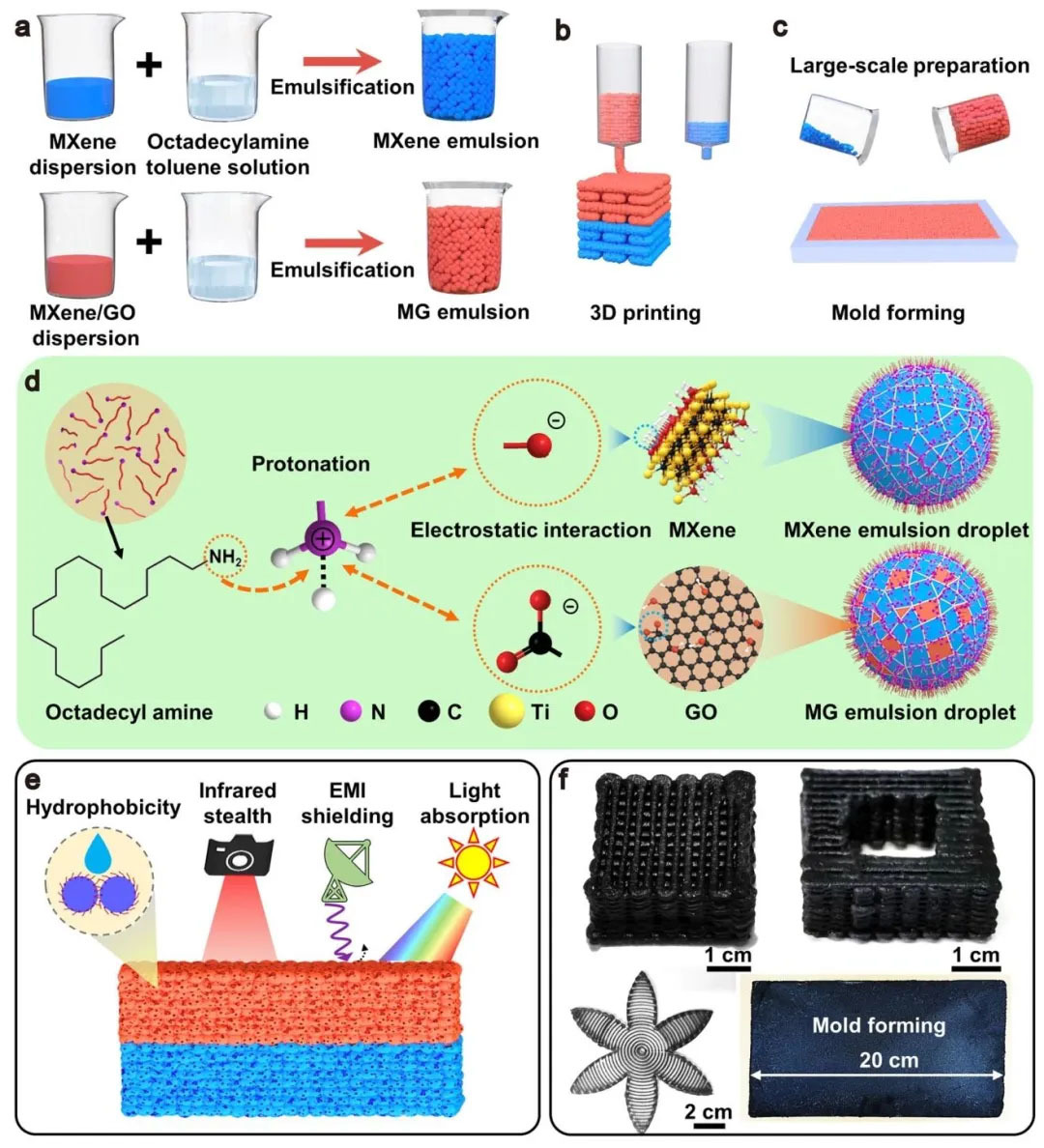
▲ Figure 1: ◦ a. Schematic diagram of MXene and MG lotion ink preparation ◦ b. 3D printing double-layer aeronenenebb gel process ◦ c. Molding large-scale preparation ◦ d. lotion interface assembly mechanism ◦ e. Schematic diagram of aeronenenebd gel versatility ◦ f. 3D printing customized shape and molded large size aeronenenebe gel photos
Figure 1: Preparation and interface assembly mechanism of multi-functional asymmetric double-layer aerogel This figure shows the preparation, 3D printing and molding process of MXene and MG (MXene - graphene oxide) lotion ink, as well as the multi-functional principle of aerogel. First, through the protonation of octadecylamine (ODA) at the oil/water interface, it forms electrostatic interaction with negatively charged MXene/GO nanosheets to generate nano surfactant and stabilize lotion drops. In 3D printing, the MG layer is printed as the impedance matching layer, and then the MXene layer is printed as the reflection layer. After freeze-drying, an asymmetric double-layer aerogel with spherical porous structure is formed. The molding process realizes large-scale preparation by pouring lotion ink into the mold to freeze dry. The porous structure of the aerogel extends the propagation path of electromagnetic waves, and combines the "absorption reflection reabsorption" mechanism to achieve ultra-high frequency absorption (X-band absorption coefficient 0.95). At the same time, it has infrared stealth, solar heating and oil cleaning functions. 3D printing can customize shapes such as petals and hollow cubes, and the molded sample size can reach 20 cm, demonstrating the flexibility of the process.
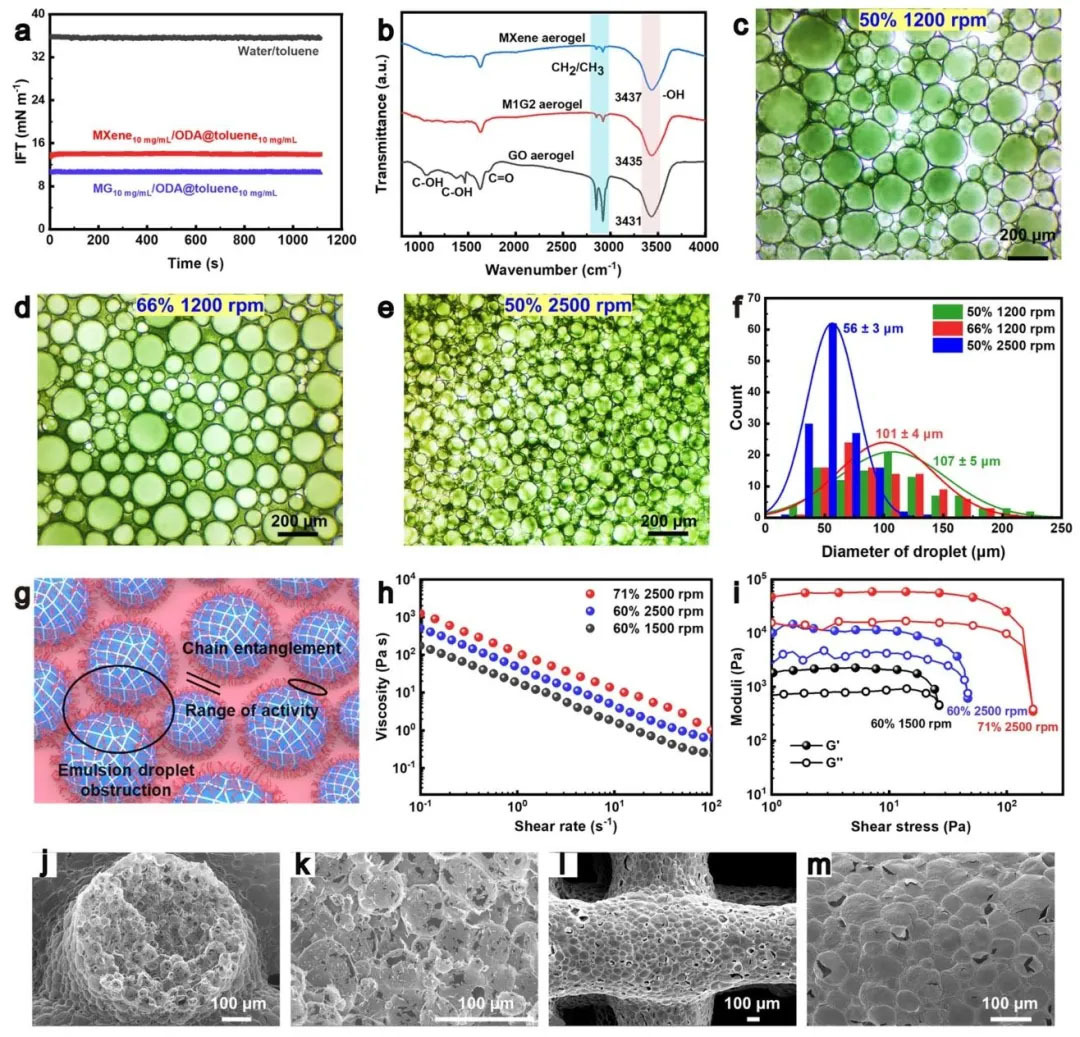
▲ Figure 2: ◦ a. Dynamic interfacial tension of different O/W systems ◦ b. FT-IR spectrum of double-layer aerogel ◦ c-e. Optical image of MXene lotion with different water phase volume ratio and mixing rate ◦ f. Size distribution of lotion droplets ◦ g. Schematic diagram of rheological property adjustment mechanism of lotion ◦ h. Viscosity shear rate curve of M1G2 lotion ◦ i. Energy storage/loss modulus shear stress curve of M1G2 lotion ◦ j-m. SEM image of MXene aerogel filaments printed (cross section and top surface)
Figure 2: Characterization of lotion interface performance and aerogel microstructure Figure 2 reveals the stability of lotion and aerogel microstructure through dynamic interfacial tension test, rheological analysis and SEM images. When MXene or MG suspension is dropped into the toluene solution of ODA, the interfacial tension decreases from 35.5 mN/m of water/toluene to 13.9 mN/m and 10.1 mN/m, confirming the formation of nano surfactants. Rheological tests showed that the mixing rate and the volume fraction of the aqueous phase could adjust the viscosity (177 – 1252 Pa ・ s) and storage modulus (2141 – 10967 Pa) of lotion, meeting the self-supporting requirements of 3D printing. SEM observed that the aerogel filaments have a spherical closed pore structure with a diameter of about 46 μ m. During the freeze drying process, the ice crystals pierce the hole wall to form secondary pores, forming a multi-level pore network, which is conducive to multiple electromagnetic wave scattering and functional integration.
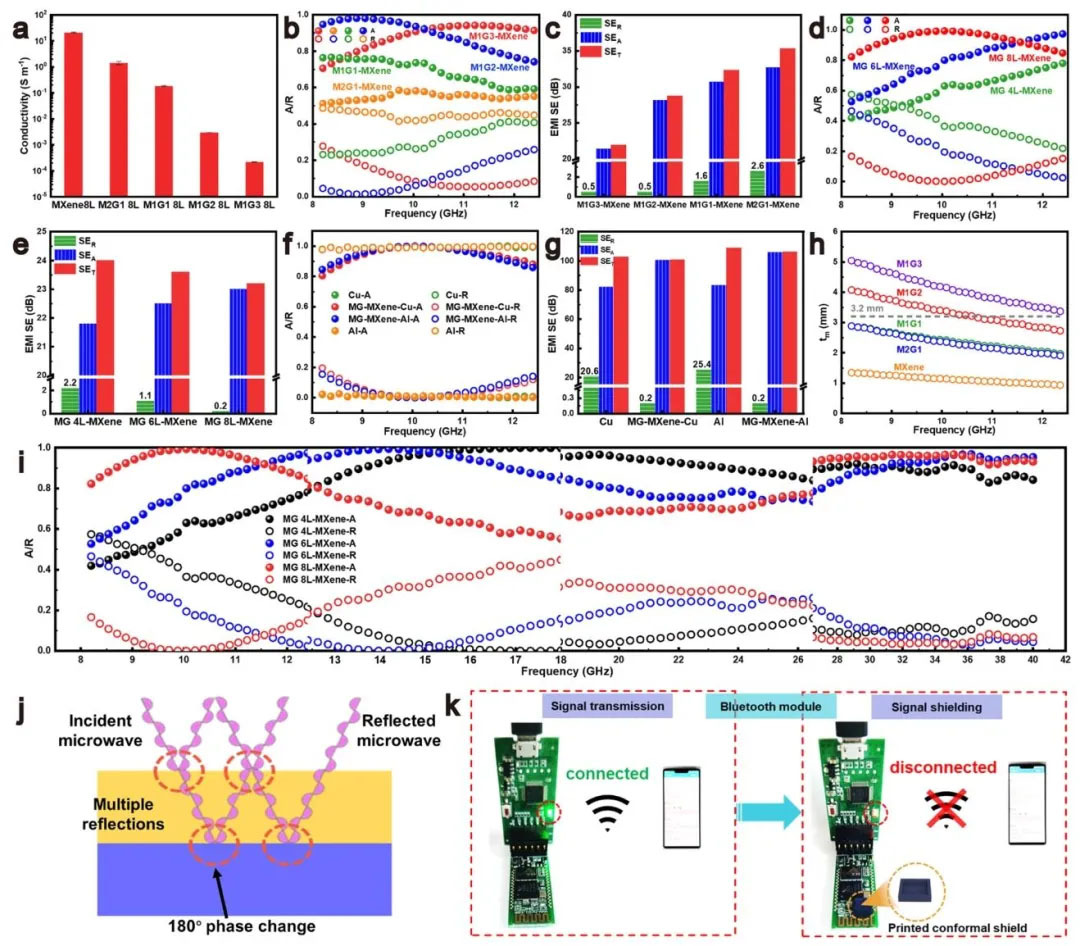
▲ Figure 3: ◦ a. Conductivity of MXene and MG layers with different GO contents ◦ b. Absorption/reflection coefficients with different GO contents ◦ c. Shielding efficiency with different GO contents (SEA, SER, SET) ◦ d. Absorption/reflection coefficients with different MG layer thicknesses ◦ e. Shielding efficiency with different MG layer thicknesses ◦ f. Absorption/reflection coefficients with attached metal foils ◦ g. Shielding efficiency with attached metal foils ◦ h. Matching thickness and frequency relationship ◦ i. Absorption coefficients with different MG layer thicknesses ◦ j. Electromagnetic wave destructive interference mechanism at the interface ◦ 9702 k. Demonstration of Bluetooth signal attenuation by 3D printed shielding cover
Figure 3: Influencing factors and optimization of electromagnetic shielding performance Figure 3 systematically studied the influence of GO content, layer thickness and metal composition on the shielding performance of aerogels. Increasing the GO content reduced the conductivity of the MG layer, optimized impedance matching, and increased the absorption coefficient of the M1G2 sample in the X-band from 0.55 of M2G1 to 0.89, while reducing the reflection coefficient to 0.5 dB. When the thickness of the MG layer was increased to 8 layers, the absorption coefficient reached 0.95, and the reflection coefficient was only 0.2 dB, demonstrating the enhancement effect of thickness matching on absorption. After the copper foil was attached, the reflection coefficient of the aerogel decreased to 0.05 while maintaining the shielding efficiency>100 dB, which verified the "absorption reflection reabsorption" mechanism. Based on the λ/4 model, adjusting the thickness of the MG layer can shift the absorption peak from 10 GHz to 17.5 GHz, achieving wideband absorption of 8.2-40 GHz.
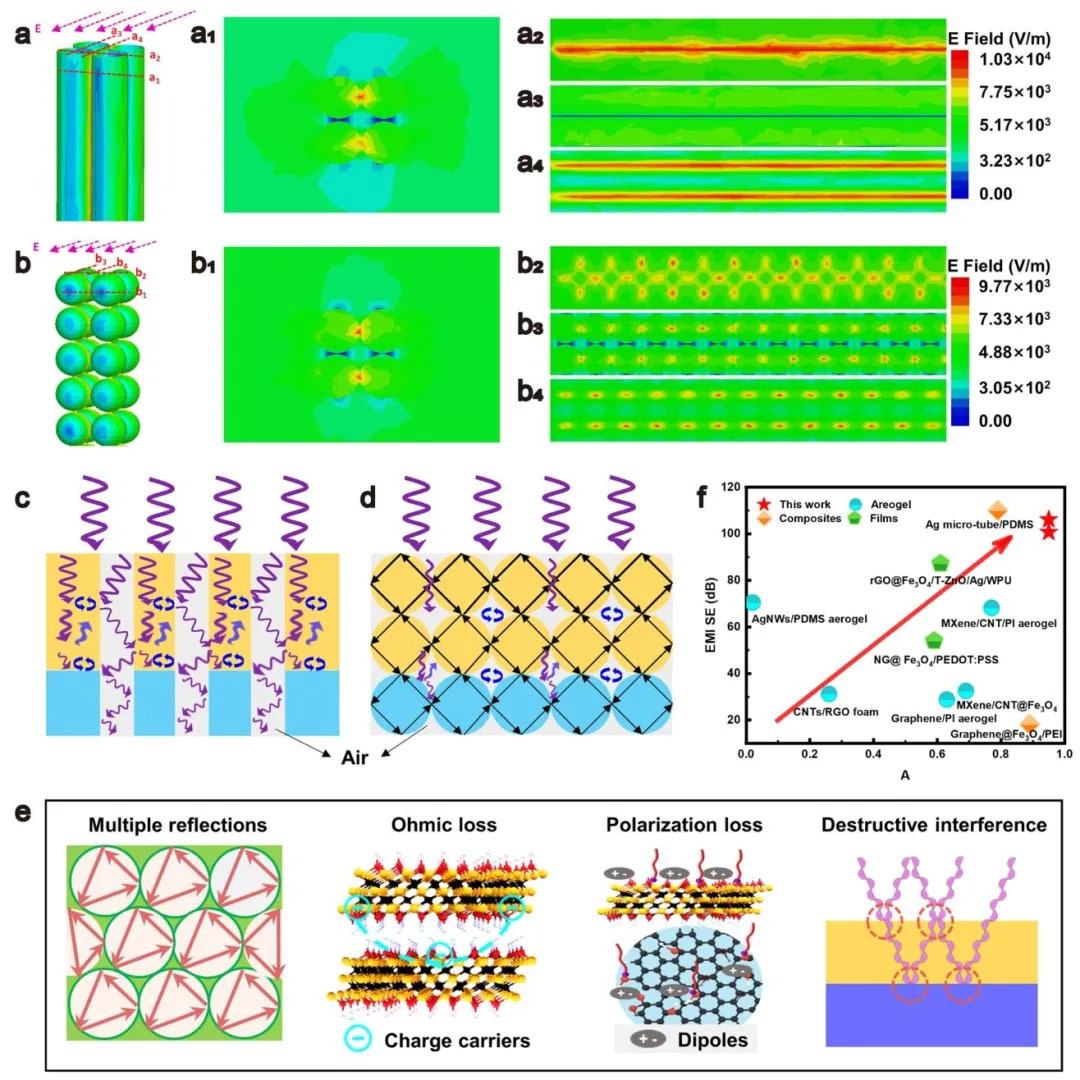
▲ Figure 4: ◦ a. Simulation of electric field distribution of straight hole gel ◦ a1-a4. Vertical plane electric field distribution of straight hole structure ◦ b. Simulation of electric field distribution of spherical hole gel ◦ b1-b4. Vertical plane electric field distribution of spherical hole structure ◦ c. Electromagnetic attenuation mechanism of straight hole structure ◦ d. Electromagnetic attenuation mechanism of spherical hole structure ◦ e. Performance comparison with other EMI materials ◦ f. Schematic diagram of electromagnetic shielding mechanism of aerogel
Figure 4: Comparison between finite element simulation and performance of electromagnetic shielding mechanism Figure 4 reveals the electric field distribution and attenuation mechanism of aerogel through finite element simulation. The electric field attenuation of spherical pore structure is more significant than that of straight pore structure, and there are strong Ohmic losses and multiple reflections at the pore interface. Through impedance matching, dielectric loss (dipole polarization of MXene/GO) and conductive loss (carrier transport of MXene), aerogels achieve an average absorption coefficient of 0.95, which is superior to Ag microtubules/PDMS (absorption coefficient 0.79) and other reported materials. In addition, the 180 ° phase difference of electromagnetic waves at the air/MG and MG/MXene interfaces leads to interference cancellation, further enhancing absorption. The porous structure prolongs the wave propagation path, allowing energy to dissipate in multiple reflections.
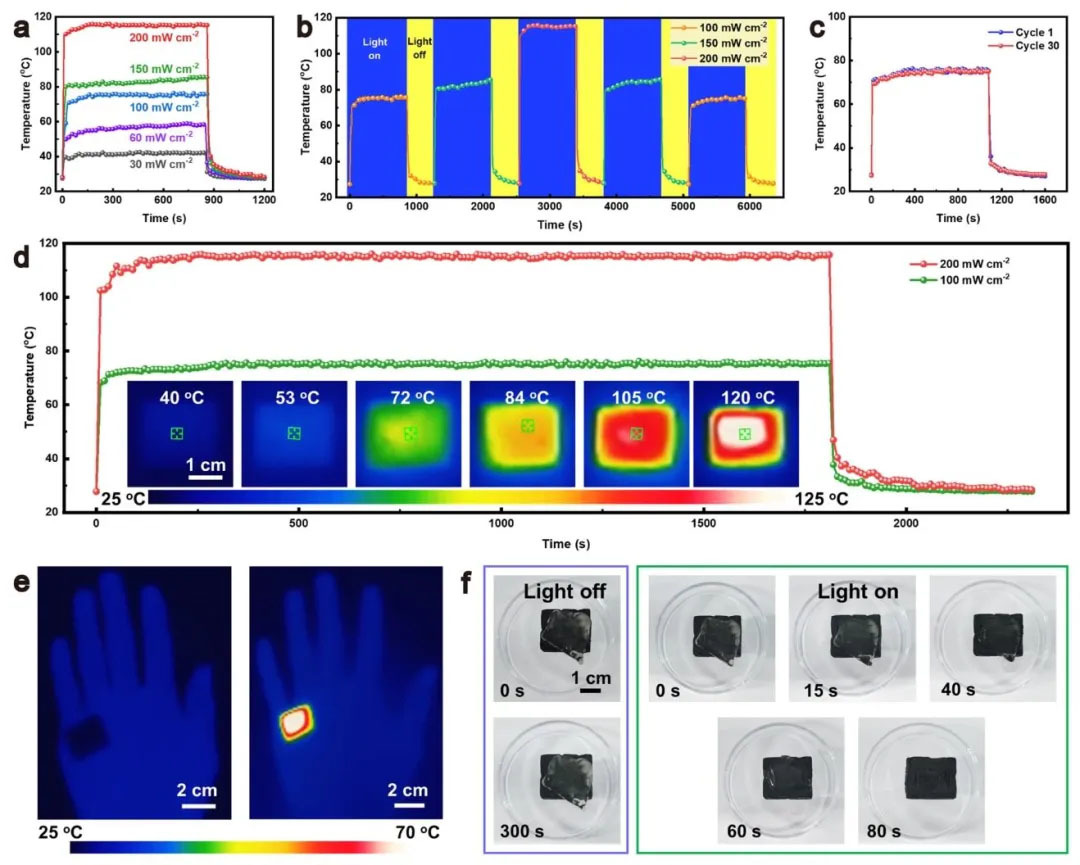
▲ Figure 5: ◦ a. Temperature time curve of gel under different light intensities ◦ b. Temperature response of gel under stepped light intensities ◦ c. Temperature stability of 30 optical switch cycles ◦ d. Temperature stability under long-term solar radiation (infrared thermal image inset) ◦ e. Infrared thermal image of solar thermal therapy ◦ f. Photo of solar radiation ice melting process
Figure 5: Solar energy thermal energy conversion performance and application Figure 5 shows that under the light intensity of 30 – 200 mW/cm ², the surface temperature of aerogel can reach 42 – 115 ℃, and the ice layer can be completely melted within 80 seconds at 150 mW/cm ², demonstrating the efficient solar energy thermal energy conversion capability. The temperature response has rapid rise/fall characteristics (heating rate ≈ 0.2 ℃/s), and the temperature stability is good after 30 cycles of optical switching, proving the reliability of the cycle. Infrared thermography shows that aerogel can be used for local thermotherapy. The surface temperature of the sample with a thickness of 1 cm is uniform under 200 mW/cm ² (temperature difference<5 ℃), which is suitable for medical and de icing scenarios.
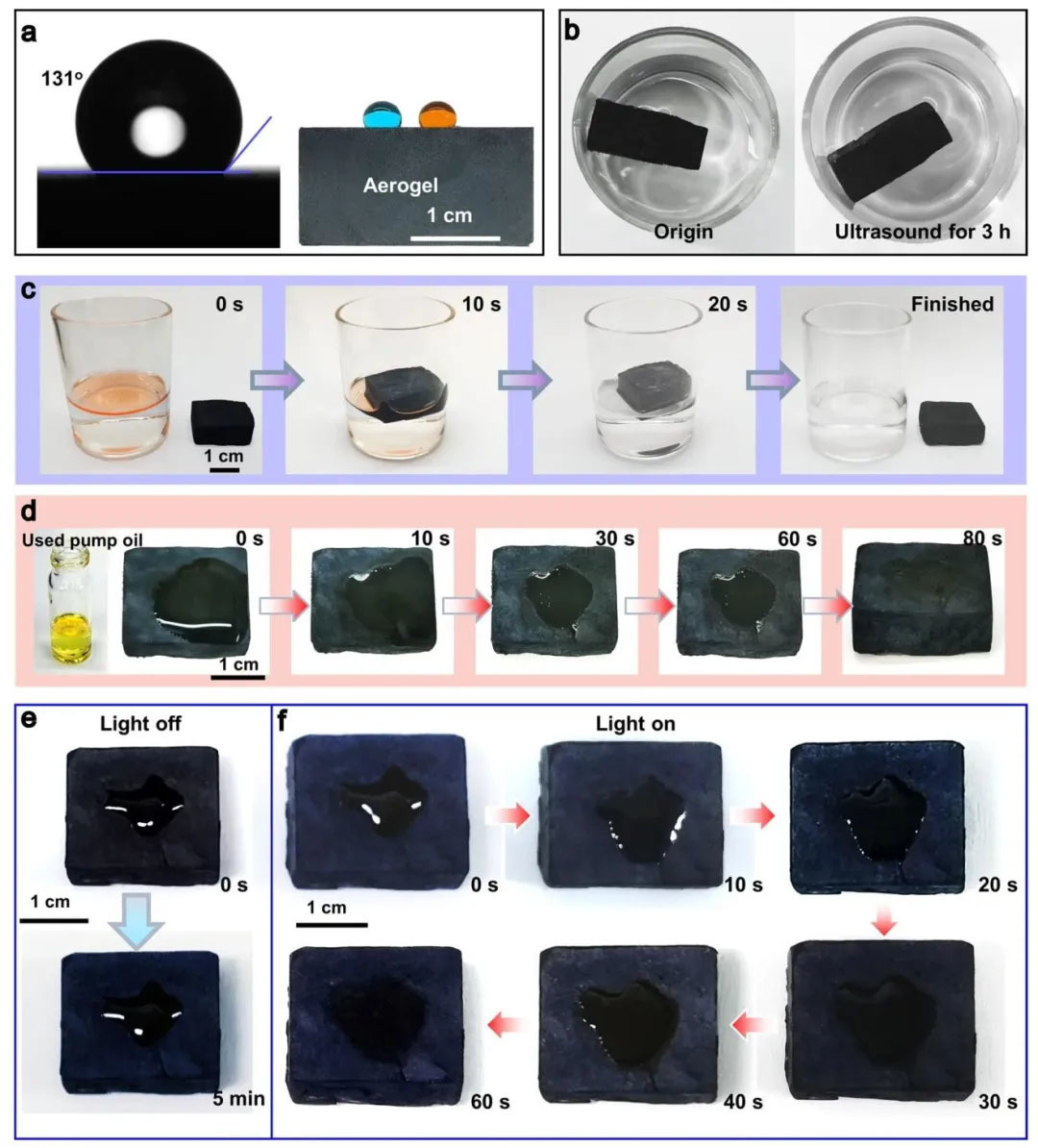
▲ Figure 6: ◦ a. Water contact angle at MXene side of aerogel ◦ b. Photos of aerogel before and after ultrasonic treatment ◦ c. Absorption process of cyclohexane by aerogel ◦ d. Waste oil absorption process by aerogel ◦ e. Crude oil absorption process by aerogel without solar radiation ◦ f. Crude oil absorption process by air pressure under solar radiation
Figure 6: Hydrophobicity and Oil Cleaning Capacity Figure 6 confirms that the water contact angle at the MXene side of the aerogel is 131 °, and the structure remains unchanged after ultrasonic treatment for 3 hours, indicating the stability of the hydrophobic surface. It can absorb cyclohexane within 20 seconds and waste oil within 80 seconds, and achieve efficient separation of cyclohexane/water mixtures stained with Sudan Red (oil phase absorption rate>99%). Under solar heating (150 mW/cm ²), the absorption time of viscous crude oil was shortened from 5 minutes under no light to 60 seconds, attributed to the decrease in crude oil viscosity caused by the increase in temperature. The hydrophobicity originates from the long-chain alkyl groups on the surface of the ODA modified MXene/GO, which makes the aerogel suitable for oil pollution cleaning at sea.
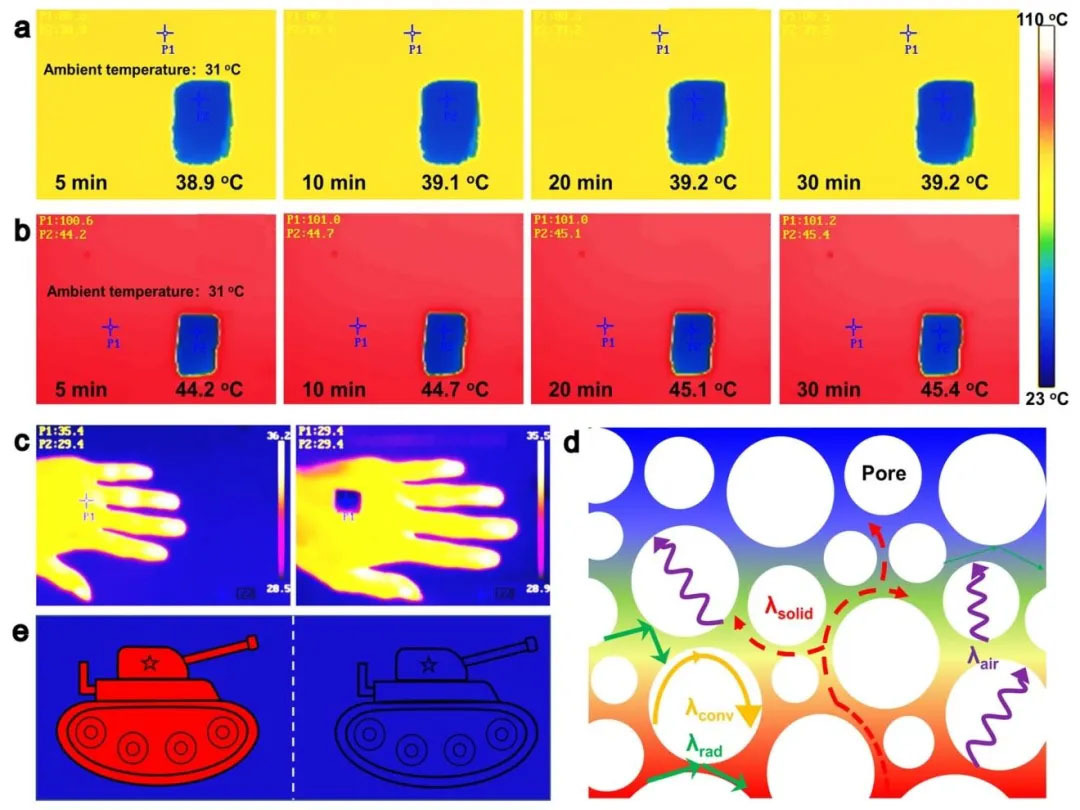
▲ Figure 7: ◦ a. Infrared thermal image of the top temperature of the aerogel on the 80 ℃ heating platform ◦ b. Infrared thermal image of the top temperature of the aerogel on the 100 ℃ heating platform ◦ c. Infrared thermal image of the aerogel covering the hands ◦ d. Schematic diagram of the thermal insulation mechanism of the aerogel ◦ e. Schematic diagram of the infrared stealth principle
Figure 7: Thermal insulation performance and infrared stealth effect Figure 7 shows that on the 80 ℃ heating platform, the top temperature of 1 cm thick aerogel is only 39.2 ℃, and the thermal conductivity is as low as 32 mW/m ・ K, proving excellent thermal insulation performance. Infrared thermal imaging shows that after covering the human hand, the skin temperature drops from 35.4 ℃ to the ambient level (29.4 ℃), achieving infrared stealth. In mechanism, the low infrared emissivity (ε ≈ 0.3) and porous structure of the aerogel inhibit the thermal radiation, and the internal air layer further reduces the heat conduction. This feature is suitable for military camouflage and thermal management fields.
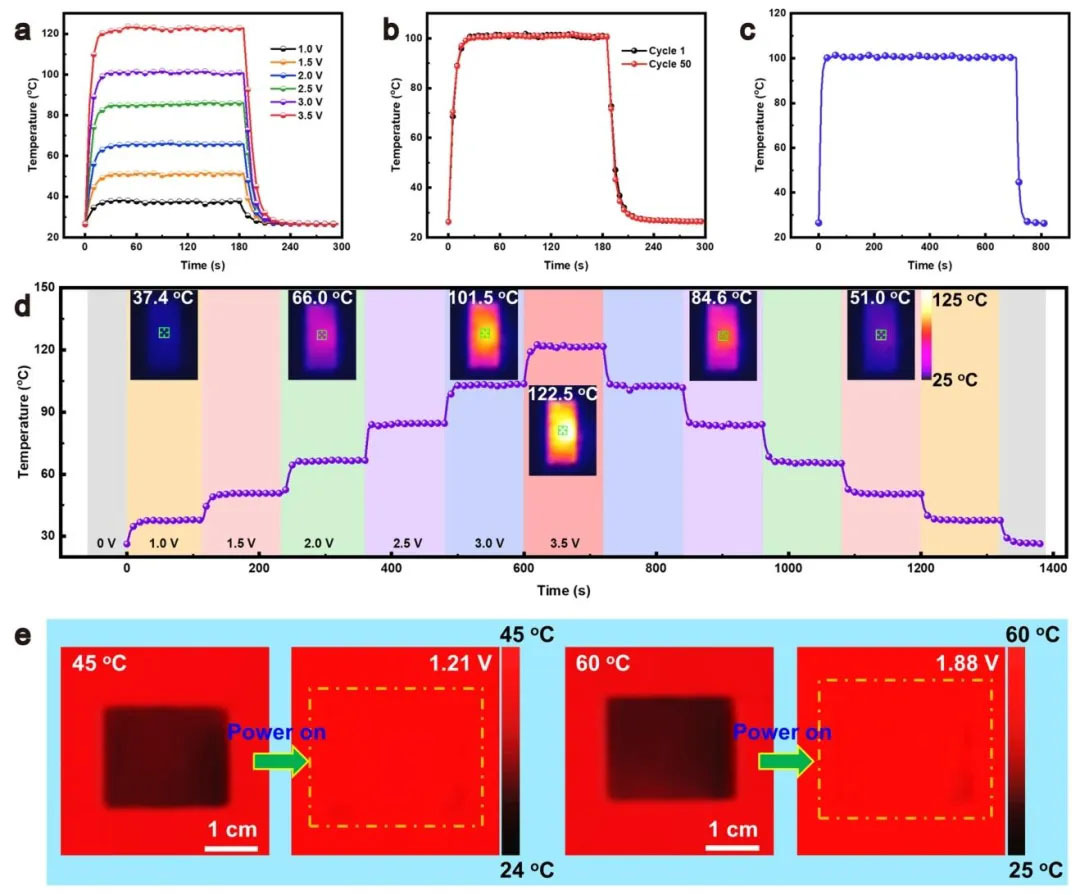
▲ Figure 8: ◦ a. Temperature at MXene side of aerogel under different voltages ◦ b. Temperature time curves of the first and 50th joule heating cycles ◦ c. Long term joule heating stability ◦ d. Cycle stability and infrared thermal image under gradient voltage ◦ e. Dynamic infrared camouflage demonstration (different voltages match the ambient temperature)
Figure 8: Joule heating and dynamic infrared camouflage Figure 8 shows that the surface temperature of aerogel can rise from 37.8 ℃ to 122 ℃ at 1.0 – 3.5 V, and the temperature fluctuation is less than 2% after 50 heating cycles, showing stable Joule heating performance. Dynamically adjusting the voltage can match the surface temperature with the environment: at 45 ℃ and 1.21 V, the aerogel infrared thermal image is assimilated with the background to achieve dynamic infrared camouflage. During the heating process, the temperature uniformity was good (1 cm sample temperature difference<3 ℃), attributed to the high conductivity of MXene and the thermal diffusion effect of its porous structure. This function expands the application of aerogel in infrared stealth technology.
Summarize
In conclusion, this paper designs an asymmetric double-layer MXene GO aerogel. Through optimizing impedance matching of the upper MG layer and enhancing reflection of the lower MXene layer, the "absorption reflection reabsorption" and interference cancellation effect are achieved. The absorption coefficient of the X-band reaches 0.95, and the broadband absorption of 8.2-40 GHz exceeds 0.9, breaking through the limitation of high reflection of traditional materials. Develop lotion ink based on ODA electrostatic assembly, which is compatible with 3D printing and molding, realize structure customization and large-scale preparation, and solve the problem of mass production of multi-functional materials. The aerogel integrates the functions of ultra high frequency absorption, infrared stealth, solar heating, oil cleaning, etc., with a density of only 10 mg/cm ³, and achieves broadband absorption regulation by adjusting the thickness of the MG layer, providing an innovative material system for electromagnetic protection and multi scene applications. Its microstructure design and multi function integration strategy provide new ideas for the research and development of similar materials.
aerogel,Aero gel


















